Progeria: When Aging Isn't Aging
How a cathedral in Venice changes our understanding of a rare genetic disease.
The Counterpoint is a free newsletter that uses both analytic and holistic thinking to examine the wider world. My goal is that you find it ‘worth reading’ rather than it necessarily ‘being right.’ Expect regular updates on the SARS-CoV-2 pandemic as well as essays on a variety of topics. I appreciate any and all sharing or subscriptions.
Hutchinson-Gilford Progeria syndrome is an ultra-rare genetic disorder. Affecting 1 in ~20 million children, there are only a couple hundred cases on Earth at any one time. Given this rarity, the disorder is unusually well known, in large part because of Sam Berns, an American boy who had progeria, was the subject of an Emmy Award-winning HBO documentary, the giver of a famous TED talk, and helped start the Progeria Research Foundation.

Progeria is universally fatal in adolescence or young adulthood due to severe atherosclerosis which eventually leads to heart attacks and/or strokes. Other symptoms include hair loss, skeletal frailty, hip dislocations, small stature, and thin and wrinkled skin.
The combination of these phenotypic traits along with the lethal cardiovascular disease in teenagers has lead to the belief that progeria is an accelerated aging disorder:
Mayo Clinic: Progeria “causes children to age rapidly”
NIH: Progeria “leads to extreme premature aging”
Wikipedia: Progeria “causes individuals to age faster than usual”
UpToDate: Progeria “is characterized by accelerated aging”
Progeria Foundation: Progeria “is a premature aging disease”
But the thing is: progeria isn’t actually aging.
Built in the heart of Venice, St. Mark’s Cathedral is an architectural marvel. Its stunning facade of arches and statues, including the Four Horses of Constantinople1, looms over the plaza and nearby Doge’s Palace. The awe continues inside with its interior of solid gold mosaics depicting many Biblical events and historical figures.


While St. Mark’s Cathedral has many notable architectural features, there is a seemingly minor one that brings us here today: the spandrel (below). These were the subject of a famous paper about evolutionary biology: “The Spandrels of San Marco and the Panglossian Paradigm,” by Stephen Jay Gould and Richard Lewontin.

On and around these spandrels are mosaics of many important figures for the Church. It’s so well put together that it almost seems designed.
To quote Gould and Lewontin: “The design is so elaborate, harmonious, and purposeful that we are tempted to view it as the starting point of any analysis, as the cause in some sense of the surrounding architecture. But this would invert the proper path of analysis. The system begins with an architectural constraint.”
Even though it might seem like the the artisans asked the architects and engineers to design the cathedral around the mosaics they had in mind, that is exactly opposite of what really happened. Round domes were very commonly used in cathedrals then and anytime you construct a round dome on top of a square base, you are going to get four spandrels. The artisans simply designed around what was necessarily constructed by the architects and engineers.
While the authors were thinking about evolution, their point has general application: sometimes the most obvious outward appearance of something isn't the reason that the underlying structure exists.
Over the last several years, I’ve co-authored a series of papers2 that provide the most robust explanation of the exact etiology of progeria.
For the vast majority of us, our LMNA gene produces an immature protein, prelamin A, that is subsequently processed into mature lamin A protein. Lamin A is one of several proteins that make up an internal lattice, the nuclear lamina (below), that provides structure and support to the nucleus, the organelle that houses our DNA.

In children with progeria, a mutation in their LMNA gene causes the post-processing of prelamin A to fail, with the end result being a toxic protein called progerin. It has been known for some time that this toxic protein leads to cell death, but not in every tissue. What explains why some tissues are affected but not others?
The insight into progeria that our papers suggest is that the cell death in progeria requires three things:
High LMNA expression/high progerin protein
Low LMNB1 expression/low lamin B1 protein
High physical forces on the cell
When all three of these conditions are met, the nuclei in specific tissues rupture causing cell death in those tissues. Thus, progeria is not actually true aging, but an architectural breakdown of the normal lamin system.
In our first paper “Disrupting the LINC complex in smooth muscle cells reduces aortic disease in a mouse model of Hutchinson-Gilford progeria syndrome,” we showed that the tissues affected in progeria (the aorta, bones, skin, etc.) have both the highest level of lamin A/progerin protein as well as lowest levels of lamin B1 protein.
Moreover, we were able to breed a strain of progeria mice that had reduced physical forces on their nuclei. We did this by altering their KASH protein, which acts as an anchor between the cytoskeleton (outside the nucleus) and the nuclear lamina (the scaffold of lamin proteins inside the nucleus). Without functional KASH, the forces from outside the nucleus cannot transmit inside the nucleus. Progeria mice without functional KASH had greatly improved aortic histology, with less cell death and less fibrosis (below).

In our second paper, “An absence of lamin B1 in migrating neurons causes nuclear membrane ruptures and cell death,” we demonstrated the importance of lamin B1 for protecting cells from nuclear membrane ruptures caused by physical forces. We did this by breeding mice that lacked lamin B1 in their forebrains.
The brain is a unique tissue because it has no lamin A (all of its LMNA expression is alternatively spliced to produce lamin C). Moreover, the migration of neurons to higher brain regions during embryogenesis depends on nucleokinesis, a process in which the cell nucleus is pulled forward by cytoplasmic motors. By studying migrating neurons in lamin-B1 deficient mice, we had cells with no lamin A or lamin B1, but were also experiencing high physical forces. This set up a situation where the cells were highly susceptible to nuclear membrane ruptures. By inserting a fluorescent protein into the cells, we could literally watch the nuclei “pop” in real time (below, panel A). The full paper has several videos of this process.
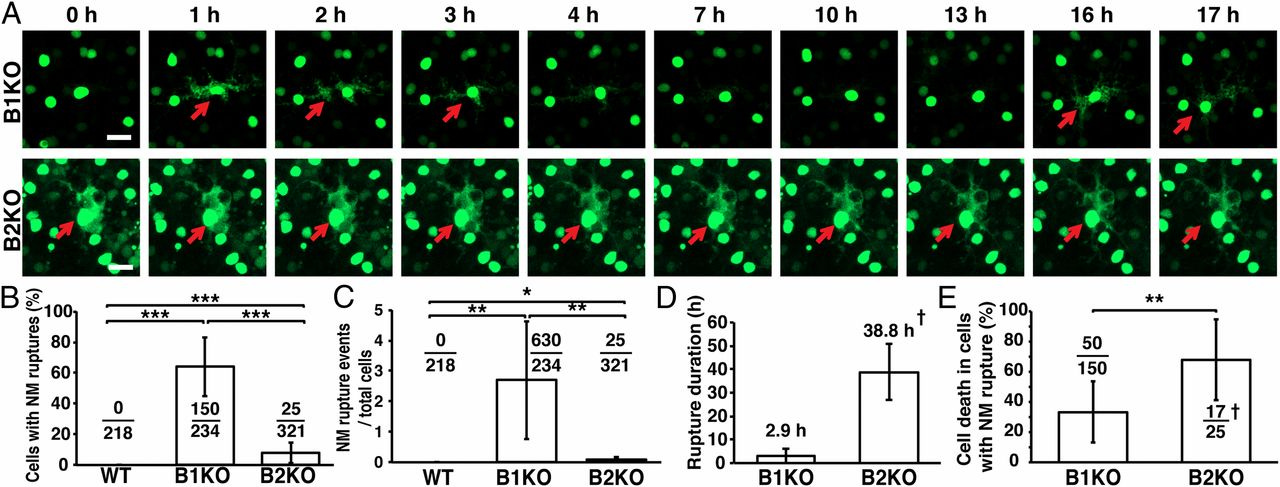
In the third paper, “Nuclear membrane ruptures underlie the vascular pathology in a mouse model of Hutchinson-Gilford progeria syndrome,” we demonstrated that lamin B1 expression can rescue cells from nuclear membrane ruptures by making them more flexible (below). Additionally, we demonstrated that the nuclear membrane ruptures in the aorta occur before cell death in progeria.

Hutchinson-Gilford Progeria is an ultra-rare and lethal genetic disease but it is widely misunderstood. Our series of papers suggest that during progeria, the dysfunctional progerin protein builds up which causes the nuclear membrane to become ‘brittle.’ If those tissues also don’t have much of ‘flexible’ lamin B1 protein, then the nuclei eventually rupture due when high physical forces are applied to them. This leads to massive cell death in certain tissues.
Just like one might mistakenly think the spandrels of St. Mark’s Cathedral were designed, rather than merely being a by-product of the underlying architecture, the unusual phenotypes of progeria (premature cardiovascular disease, skeletal fragility, hair loss, thin and wrinkled skin, etc.) have been mistaken as premature aging, rather than the underlying architecture of the lamin system that provides critical structure to cell nuclei. To analogize, sometimes old buildings collapse. But if a newly constructed building collapsed because low-quality mortar was used, we wouldn’t call it old.
Children with progeria aren’t aging faster, they are breaking down in a way that just so happens to resemble aging.
The actual horses were moved inside long ago for preservation purposes, but even the replicas are stunning.
Most of the credit should go to my co-authors; they are the true experts. I’m simply a generalist that has managed to find himself in interesting places.

Thanks for the extremely clear and convincing explanation of your research.