Should You Take the New Bivalent Vaccine?
The new bivalent vaccine updates, strengthens, and broadens one's immunity. They are safe with minimal side effects. While you should very probably schedule one, there are some considerations.
The Counterpoint is a free newsletter that uses both analytic and holistic thinking to examine the wider world. My goal is that you find it ‘worth reading’ rather than it necessarily ‘being right.’ Expect regular updates on the SARS-CoV-2 pandemic as well as essays on a variety of topics. I appreciate any and all sharing or subscriptions.
On August 31st, the FDA amended the emergency use authorizations (EUA) of both the Pfizer and Moderna vaccines to authorize bivalent formulations of each.
These bivalent vaccines are exactly the same as the previous formulations except that their mRNA is now a 50:50 mixture between the original mRNA and new mRNA that codes for the spike protein of Omicron BA.4 and BA.5 (below, highlights mine).1 These two strains currently represent ~89.7% of cases in the United States.
One of the challenges with public health is that health, by it’s very nature, is individual. It is hard to apply simple and broad rules to a large and diverse society in which everyone’s situation is unique. What is clear is that the bivalent vaccines are safe and bolster one’s immunity to SARS-CoV-2, reducing the likelihood of infection, disease, death, and Long COVID.
While you should probably schedule an appointment for the bivalent vaccine, it isn’t for absolutely everyone. The purpose of today’s newsletter is to explain why you should take the bivalent vaccine, who is eligible, and when to schedule the appointment.
The Bivalent Vaccine Works
The bivalent vaccine strengthens and broadens your immunity.
Dr. Jacqueline Miller handled the Moderna presentation at the last Advisory Committee on Immunization Practices (ACIP) meeting2. Below is one slide from that presentation. It shows that the BA.5 bivalent vaccine (purple) substantially increases neutralizing antibodies against both Omicron BA.1 (top row) and Omicron BA.5 (bottom row), with a similar result is seen with a BA.1 bivalent vaccine (orange). Note the log scale on the Y-axes.
Dr. Kena Swanson handled the Pfizer presentation at the last ACIP meeting. Below is one slide from that presentation. It shows that the BA.5 bivalent vaccine (purple) substantially broadens neutralizing antibodies, increasing them against all Omicron variants tested (BA.1, BA.2, BA.2.12.1, BA.4, and BA.5). Note the log scale on the Y-axis.
This strengthening and broadening of immunity has been verified by independent researchers, for example this preprint study, which was led by Dr. Michael S. Diamond from Washington University School of Medicine. Dr. Jesse Bloom of the Fred Hutch Institute did a helpful breakdown of the study on Twitter.
Now, you might have noticed that both these presentations and the independent study relied heavily on data from mice. Yes, this is true as the science and regulation is following a similar process that updates the influenza vaccine on an annual basis. This includes running human trials, which take longer, in parallel with the aforementioned mice trials, which produce data more quickly3.
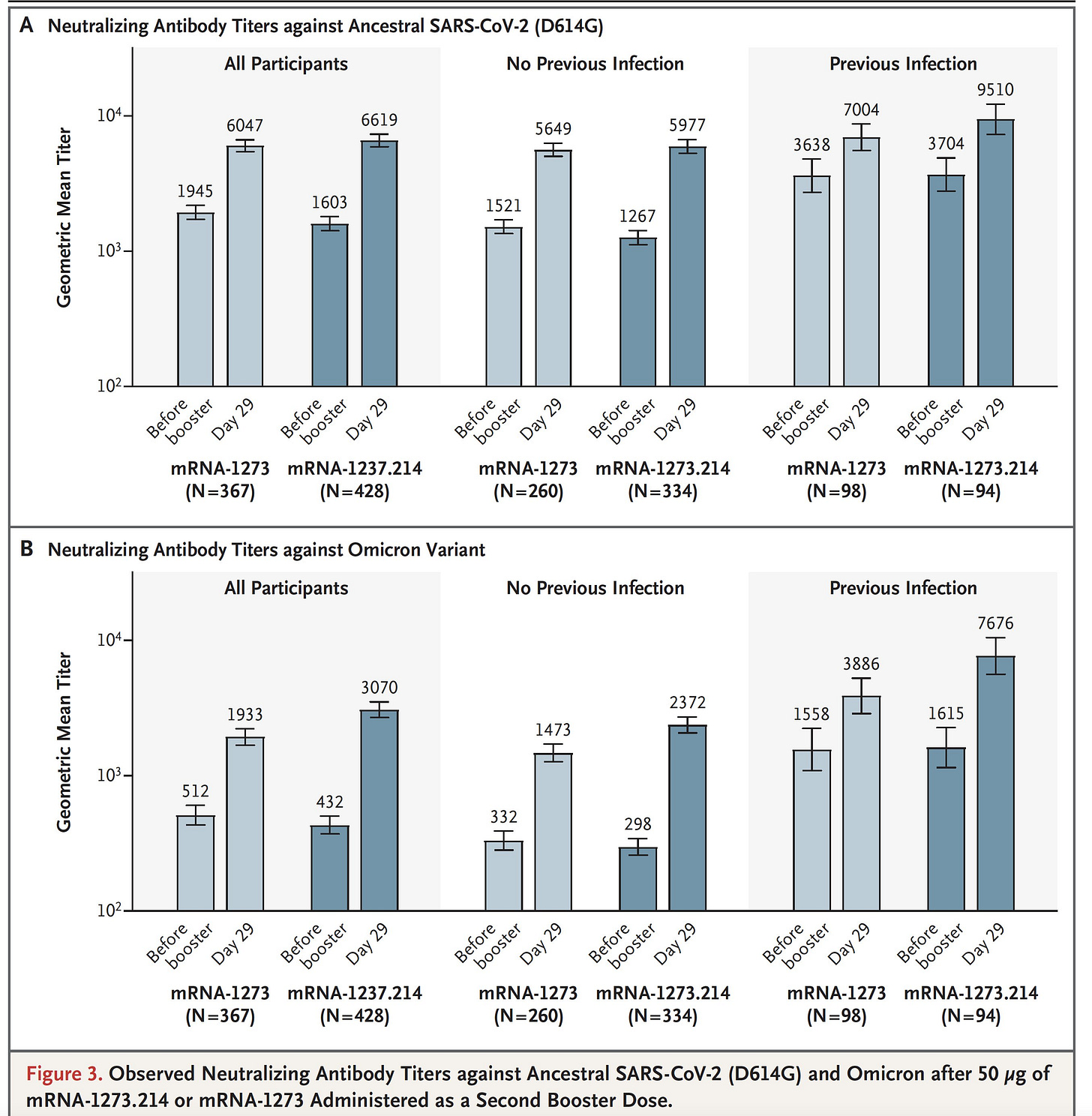
Fortunately, on September 16th, Moderna published the first human data with the BA.5 bivalent vaccine. Below is Figure 3 from the paper showing that bivalent vaccine (the dark blue) substantially increasing neutralizing antibodies in patients with or without a history of previous CoV-2 infection. Note the log scale.
Bottom line: the new bivalent vaccines update, strengthen, and broaden one’s immunity.
The Bivalent Vaccine Is Safe
The bivalent vaccine is safe with minimal side effects.
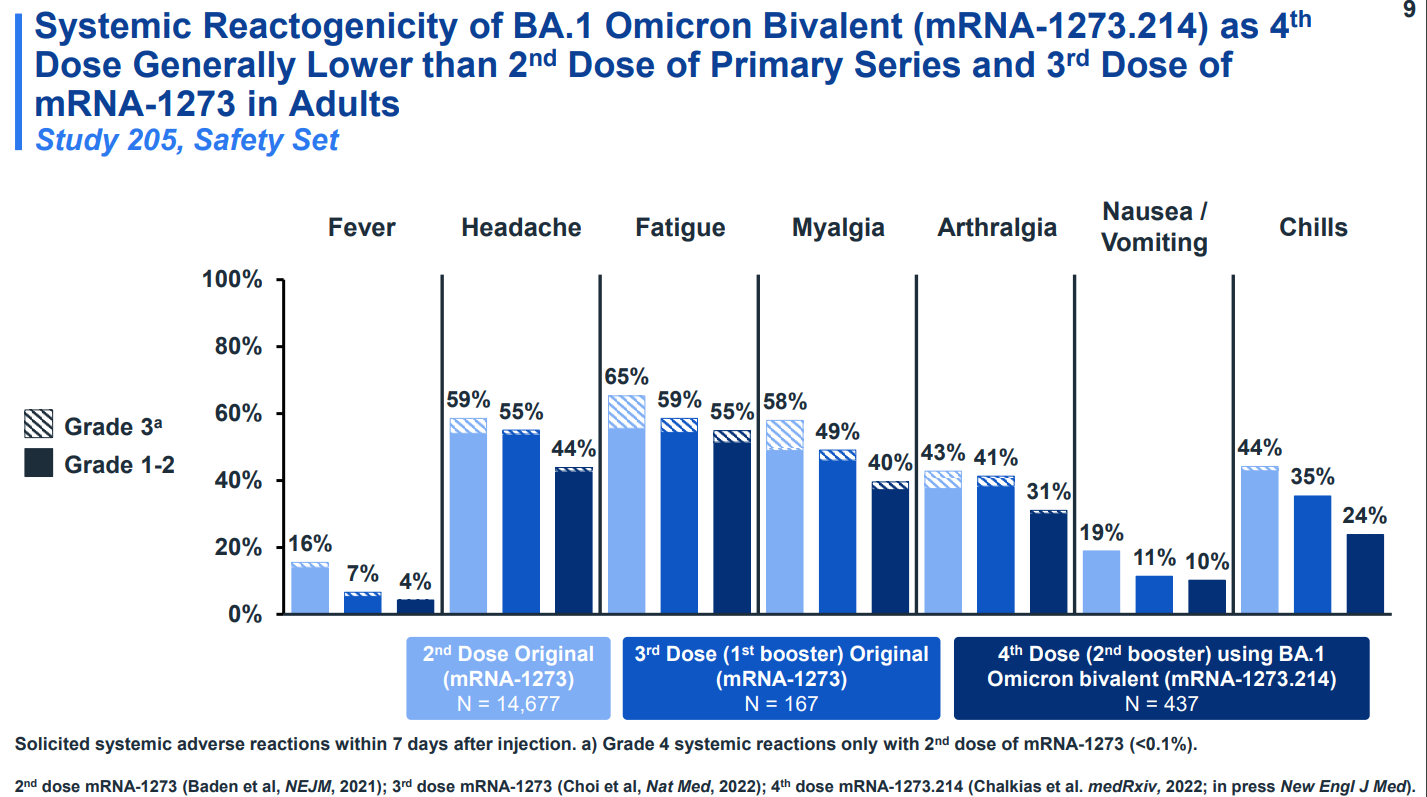
A bivalent vaccine (darkest blue) showed fewer side effects than either the second or third dose (lighter blues) of the original vaccine. Almost all side effects were mild or moderate. None were life-threatening. The below image is from the aforementioned Moderna presentation at the ACIP meeting.
Bottom line: the bivalent vaccines are safe.
Who Should Take the Bivalent Vaccines and When Should They Take Them?
The FDA’s authorization for the new vaccines is age-gated. The Pfizer bivalent vaccine is authorized for ages 12+ and the Moderna bivalent vaccine is authorized for ages 18+. Those under the age of 12 are currently ineligible for the bivalent vaccine.
Additionally, the authorization is time-gated. If you have either tested positive for COVID-19 or received a COVID-19 vaccine within the last two months, then you are not eligible for the bivalent booster. This is because research, such as this preprint, suggest that vaccination after recent infection or recent vaccination provides less benefit than waiting a few months.
This makes sense at a high level. A recent infection or vaccination will induce a high level of antibodies against SARS-CoV-2. These antibodies will remain high for a couple months and slowly wane over several months to much lower levels. Taking a vaccine during the ‘high antibodies’ phase will add less protection than if taken during the ‘low antibodies’ phase.
Moreover, while the FDA’s official recommendation is at least two months after the last infection or vaccination, I would not interpret that as set in stone. If possible, I would try to delay it between three to six months. If you are high-risk, aim for closer to three months. If you are low-risk, aim for closer to six months. If it has been more than six months since your last vaccination or infection, schedule an appointment ASAP.
Finally, as discussed in a previous newsletter, our immune systems have a circadian rhythm. Multiple studies have found stronger immune responses were elicited with morning vaccination of COVID-19, BCG, and influenza vaccines. This results suggest scheduling your vaccination before 12 noon for the strongest response.
Bottom line: The bivalent vaccine is recommended for everyone over the age of 12 if it has been 3+ months since your previous infection or vaccination. Scheduling your vaccine in the AM may induce a stronger immune response.
Myocarditis
Myocarditis is inflammation of the heart tissue. It has been the side effect from the mRNA vaccines that has received the most media attention and for good reason, it can be quite serious. Post-vaccination myocarditis has been mainly observed in adolescent males.
Dr. Tom Shimabukuro presented the data on vaccine safety at the ACIP meeting. Below is the slide for the rates of myocarditis in males and females following the 2nd dose and the booster dose. All peach-shaded boxes indicate rates above the background incidence of myocarditis in the general population. The rate peaks for 16-17 year old males at 78.7 cases per 1 million vaccinations with the 2nd dose.

While this is low, it is worth considering. As discussed in the previous newsletter “mRNA Vaccines and Myocarditis,” COVID-19 itself carries a risk of myocarditis. According to Patone et al., the rate of myocarditis in those <40 years old following COVID-19 is ~10 per 1 million. While this is higher than the vaccination-myocarditis risk for several groups, it still means that adolescent males face more risk of myocarditis from vaccination than from COVID-19.
But also discussed in the previous newsletter is that there are many more risks from COVID-19 than just myocarditis. In the cardiovascular system alone, COVID-19 can also cause pericarditis and cardiac arrythmia. Outside the cardiovascular system, COVID-19 can damage nearly every organ system acutely, cause Long COVID chronically, and, obviously, even kill you. Yes, the rates for all of these are low in younger people. But so is the rate of myocarditis post-vaccination (at least I find 78.7 cases out of 1 million to be low). My point is that the total risk from COVID-19 remains higher than the total risk from vaccination regardless of your sex or age bracket.
Additionally, notice the second half of that slide. The rates of myocarditis went down for every group with the 1st booster compared to the 2nd shot of the primary series. This is believed to be because of the timing between doses; the 2nd shot was only 2-3 weeks after the 1st shot, while the 1st booster was many months after the 2nd shot. This is one of the reasons that I suggested healthier people wait until 5-6 months after infection or vaccination for the bivalent booster. Such spacing will almost certainly reduce the incidence of serious side effects.
Bottom line: Myocarditis is a risk for adolescent males following vaccination. However, COVID-19 presents more total risk and a cost/benefit calculation suggests vaccination remains beneficial.
Finally, while I hope I’ve convinced you of the safety, effectiveness, and importance of the new bivalent vaccine, I know that data and scientific slides can be abstract and cold.
My wife and I are both 30 years old. My wife also happens to be ~20 weeks pregnant with our firstborn. As a soon-to-be father, I would never do anything to endanger my family. While neither of us are at high-risk, or even moderate-risk, from COVID-19, it is clear to me that SARS-CoV-2 presents much more risk to my family than an vaccine that has been given to billions of people.
I want to protect my wife, my future newborn, my parents, my in-laws, and myself from the virus that has killed over 1 million Americans. This is why I’ve scheduled an appointment for both my wife and I to receive the new bivalent vaccine in early October. I hope you’ll schedule one as well.
Omicron BA.4 and BA.5 have identical spike proteins. The mutations that distinguish them are all in CoV-2’s other proteins.
There was human data presented at the ACIP meeting, however, it was with a BA.1 bivalent vaccine, rather than a BA.5 bivalent vaccine.